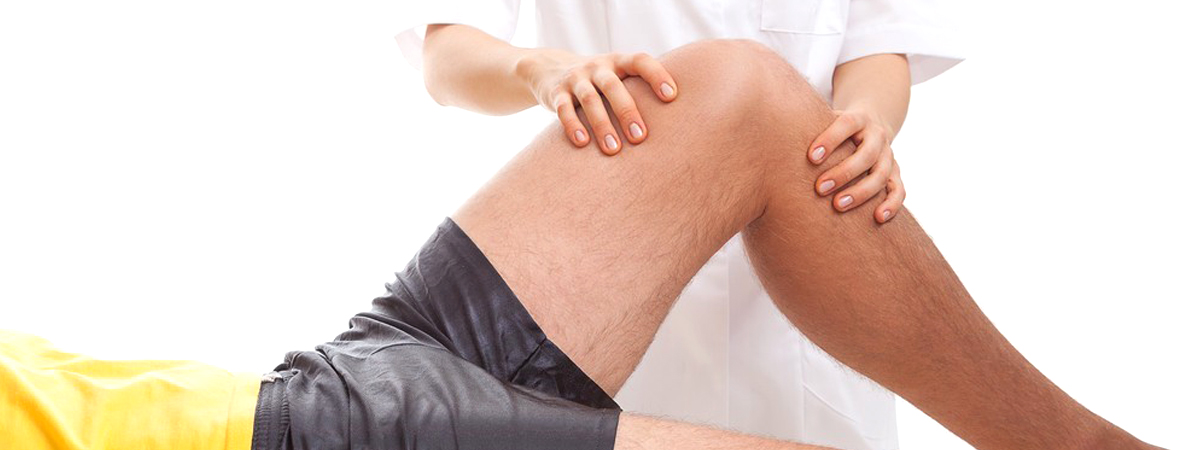
Supartz joint fluid therapy has been used for over 20 years around the world. It was FDA approved in January of 2001 for osteoarthritis in the knee. It’s indicated for treatment of pain in osteoarthritis to restore the cushioning and lubricating properties of normal joint fluid and thereby prolonging the life of damaged or arthritic joints. It’s a sterile viscoelastic non-pyrogenic solution of purified, high molecular weight sodium hyaluronate which is extracted from chicken combs. The chicken combs are harvested from chickens that have been certified as suitable for human consumption via pre and post mortem veterinary inspection.
Supartz is generally used after conservative treatments have failed, such as using anti-inflammatory medications, exercise or physical therapy and is used as a great alternative to surgery for many reasons, it is much less invasive, much less expensive and can be performed in an office setting. The injections are usually given intraarticularly once a week for a series of 5 weeks, but many patients are treated within just 3 injections. The entire procedure should take no more than 15 minutes to perform. In aiding with shock absorbation and lubrication, Supartz helps the joint move smoother, thereby lessening pain and improving mobility.
Contraindications to Supartz include those patients who have hypersensitivities to sodium hyaluronate preparations as well as avian proteins, feathers and egg products, patients with skin diseases or infections in the area of the injection site, and Supartz has not been clinically tested for safety and effectiveness in pregnant or nursing women as well as in the pediatric population. Disinfectants containing ammonium salts should not be used to prepare the skin prior to injections because it can cause the sodium hyaluronate to precipitate. Excess joint fluid should be removed prior to the injections via arthrocentesis.
Pain or swelling may occur at the injection site post-injection. It is recommended that the patient avoid strenuous activities or prolonged weight bearing (more than 1 hour) after the 1st 48 hours post injection.
A Clinical study was done on Supartz which was randomized, multi-centered, blinded and placebo controlled. 5 weekly injections of the knee were given in all of the studies. Pain was found to be the most common side effect, which was reported in 18.4% of the patient population that received Supartz, as well as 16% of those that were the control patients. Arthralgia was the second most common side effect, reported in 12.6% of Supartz patients and 15% of controlled patients.
In most cases, Supartz injections of the knee for the treatment of osteoarthritis Is covered by insurance, but it is often not covered by insurance carriers for treatment of arthritis in the foot and ankle. It is used off label in these locations and is currently not FDA approved in these areas.